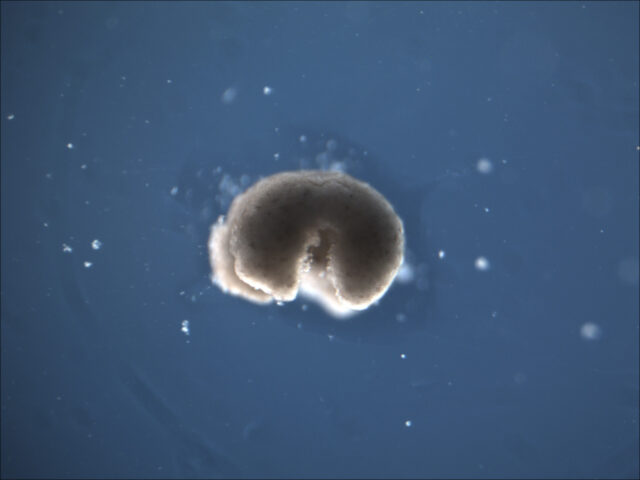
By Kathryn Nave, contributor
Robots may build cars and assemble computers, but the one thing they can’t yet rebuild is themselves. Computer scientist Sam Kriegman wants to change that. After having limited success with the traditional materials of plastic and metal, Kriegman had a meeting with developmental biologist Douglas Blackiston in 2019 and came up with an idea: Rather than starting from scratch, why not build a robot out of something that can already repair and replicate itself? Why not use living cells?
“Looking at a cell is like being presented with some kind of futuristic technology,” says Kriegman. “It has so much machinery that we just don’t understand yet and so much adaptive capability. When you figure out how to build with this seemingly magical material, you get a lot of amazing properties like self-repair, replication or biodegradability that are very difficult to achieve in current robots.”
At the time, Kriegman, now an associate professor at Northwestern University, was a postdoctoral researcher at the University of Vermont, while Blackiston is a senior scientist at Tufts University. Together with their collaborators, Tufts biophysicist Michael Levin and University of Vermont computer scientist Josh Bongard, they started looking into how the engineering technologies created in the field of synthetic biology might allow them to create cellular robots.
Engineering biology
In the late 20th century, developments in DNA sequencing technology opened a window into how the genetic code controlled a cell’s responses to its environment. Synthetic biology involves hacking into these genetic and regulatory networks in order to create desired patterns of behavior. Such techniques can be deployed to assemble groups of cells into tiny biological-artificial hybrid machines, like the artificial stingrays and jellyfish created by Harvard University Bioengineering Professor Kevin Kit Parker, that could one day be utilized for tasks such as targeted-drug delivery or environmental clean-up.
Still, to Kriegman and Blackiston’s eyes, much of this work suffered from two major limitations that they wanted to improve upon.
First, the designs were typically constrained to the replication of familiar biological structures rather than the exploration of novel designs. Second, the resulting bio-machines were usually built onto a synthetic scaffold, which lacked the properties of biodegradability and self-repair that would be particularly desirable for medical deployments inside the human body.
When you figure out how to build with this seemingly magical material, you get a lot of amazing properties like self-repair, replication or biodegradability that are very difficult to achieve in current robots.
—Sam Kriegman, associate professor, Northwestern University
When Kriegman described his work on using evolutionary algorithms to generate novel robot designs, Blackiston replied that he thought he could make those designs entirely out of nothing but frog cells, and the Xenobots project was born.
“Everyone thought Doug was crazy, but once he’d shown that the shapes that the algorithm came up with could be built from cells, we were off to the races,” Kriegman says. “I completely dropped everything I was working on, and we started really focusing on this.”
Artificial evolution
Just as the Xenobots material is biological, so, too, is the inspiration behind the design process. As with evolution by natural selection, Kriegman’s algorithm randomly generates a variety of possible forms, ranging from dumbbells and rings to Pac-Man-shapes to something that looks a bit like a squished marshmallow or a molar tooth.
These virtual Xenobots are then simulated in a physics engine and evaluated against how well they perform some behavioral goal, such as walking or swimming. The least successful designs are deleted, the best ones undergo some further random modifications, then they compete again. The process is repeated until the algorithm settles on a structure that seems promising enough to build with real cells in the laboratory at Tufts University.
Because Kriegman initially used the same algorithm he’d been deploying for the design of mechanical robots, and because there was little knowledge available about how cells will behave in artificial configurations, the chosen designs were initially either impossible to build or behaved completely different than expected.
“We had no idea what heart cells would do if they were in a shape other than an adult heart,” he says. “For instance, if you had two clusters with a thin bridge between them, like a dumbbell, would they synchronize their contractions? The experts had no idea. That’s very different from building with metal and motors where we can predict pretty much exactly how the materials will behave.”
It turned out that the heart muscles did synchronize—a fact that Kriegman and Blackiston have now incorporated into their computational model for future designs. By going back and forth between simulated designs and real-world constructions in this way, they’ve been able to iteratively improve their model to the point that it is now able to produce much more reliable predictions of how the cellular constructs will behave.
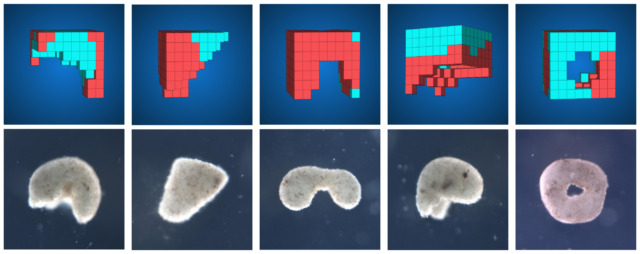
“Humans have made all sorts of amazing machines, but this still just covers the tiniest region of the space of all designs that could possibly exist,” Kriegman says. “We’re really biased in our imaginations and limited our own experience. By evolving these designs inside a computer, we’re able to expand our imaginations to create all of these crazy designs that a human might not think of.”
Cellular Lego
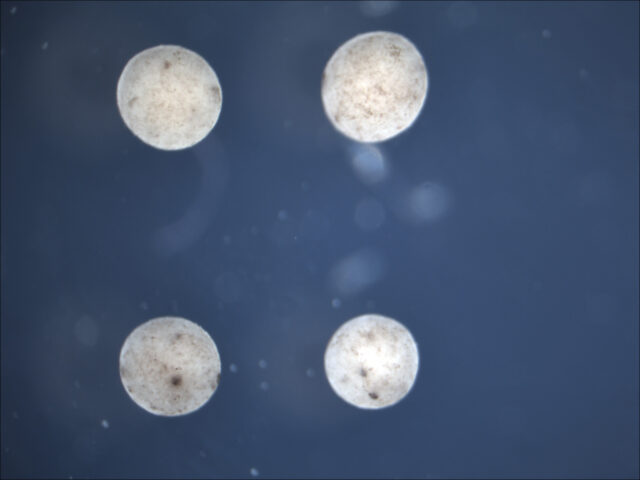
Once the evolutionary algorithm has selected a design that performs well in the computer simulation of a particular challenge— for instance, a Pac-Man-shaped Xenobot that was most effective at clearing particles in its environment—Doug Blackiston then assembles it in reality. The first step is harvesting the basic materials from a frog embryo. These consist of skin cells to build the body of the Xenobot and heart muscle cells to power the contractions that generate its walking motion.
“Within the first two days of development, you can sort of treat the embryo like a globe, where if you look around the surface, there’s certain parts or regions that correspond to different continents,” Blackiston explains. “In the case of the embryo, that means there’s an area where all the cells are fated to be skin cells, an area that will be neural cells, and so on. That allows you to deconstruct it and put each of these into little piles like different types of Lego blocks.”
The assembly process itself is partially a matter of layering these cells on top of one another, using a very steady hand and an array of micromanipulation devices. Yet the cells will also do much of the assembly work themselves.
“Cells tend to self-organize into patterns,” Blackiston describes. “You can imagine it like chess pieces that automatically return to their places when you mix them up. That’s why 99% of the time, if you start with a frog embryo, it will form into a functional frog.”
By using a variety of stimulation methods, ranging from particular chemicals and colored lights to the insertion of small sections of genetic material, Blackiston is able to subtly shift those patterns, coaxing the cells into his desired configuration.
“Instead of having to place every cell into a pattern by hand, I can take the natural pattern and deflect it,” he explains. “So, if you imagine them as automatically forming a checkerboard, for instance, there are tricks I can use to divert that process so they would form up with all the black pieces on one side and all the white pieces on the other instead.”
Smarter Xenobots
For Kriegman and Blackiston, some of the most exciting things about the Xenobots are the questions they raise about how to think about life and the insights they provide into how to manipulate the plasticity of biological systems.
“We’re rearranging these cells into something that moves like a single unit but is clearly not a frog,” Kriegman says. “Yet if you zoom in, these are 100% frog DNA. The Xenobot is something living, but it’s also something that’s been artificially created. So, it’s this interesting object where you’re not sure whether it’s really an organism or whether it’s really a robot.”
The Xenobots have already surprised their creators by demonstrating capacities such as the ability to repair themselves to restore function when damaged. Last November, the team demonstrated that they were even capable of a form of replication—achieved by pushing together unattached frog cells in their environment into more sphere-shaped Xenobots.
Currently, however, they move about randomly due to the lack of any sensors that would enable them to respond to their environment. Now, Blackiston is working on equipping the Xenobots with simple sensorimotor loops via different types of sensitive proteins, which can be implanted into muscle cells to cause a contraction in the presence of light or a particular chemical.
By making it possible to actually control where the Xenobots go, this could turn what is currently a novel demonstration of biological plasticity into something with real-world applications.
“When machines with artificial parts reach the end of their lifecycle, they end up leaving trash in the environment, but these are completely biodegradable, which makes them ideal for environmental or healthcare tasks,” Blackiston says. “We might one day be able to build Xenobots out of a patient’s own cells, for instance, and have them carry a drug to the place in the body where it’s needed. Our hope is that work is the first step in what will become a new research program in the creation of fully biological machines.”
Lead image of a manufactured organism with four limbs courtesy of Sam Kriegman and Douglas Blackiston.